
CX 2025: Bioresorbable scaffold technology has ‘potential to be a new paradigm for femoropopliteal endovascular intervention’
cardiovascularnews.com
May 5, 2025, 6:10 a.m.
In Wednesday’s peripheral arterial programme, which hosted several podium-first presentations, lower limb drug-eluting technologies took centre stage—signalling a potential shift in the treatment paradigm for chronic limb-threatening ischaemia (CLTI). Investigators shared compelling new data from studies ranging from first-in-human trials to large-scale registries, which vie to redefine durability and safety, as well as cost-effectiveness, in the peripheral arterial treatment landscape.
Share on

How is Critical Limb Ischemia treated?
aadicura.com
March 17, 2025, 8:22 a.m.
Critical Limb Ischemia (CLI) is a condition manifested by pain at rest, a non-healing wound, or gangrene. It occurs due to severe blockages in the arteries of a person’s lower extremities/limbs (legs), which markedly reduces blood flow. This condition is a serious form of Peripheral Arterial Disease or PAD that is caused by the buildup of fats, cholesterol or some other substances in and around your artery walls.
Share on
Two-year LIFE-BTK data show sustained benefits of drug-eluting resorbable scaffold for below-the-knee arteries
cardiovascularnews.com
Dec. 7, 2024, 6:30 a.m.
Presented today, late-breaking data from the second year of the LIFE-BTK clinical trial demonstrate the long-term effectiveness of the US Food and Drug Administration (FDA)-approved Esprit BTK everolimus-eluting resorbable scaffold system (Abbott Vascular) in patients with the most severe form of below-the-knee (BTK) peripheral arterial disease (PAD). The data show that the Esprit BTK offers sustained benefits over balloon angioplasty with fewer repeat procedures at two years.
Share on
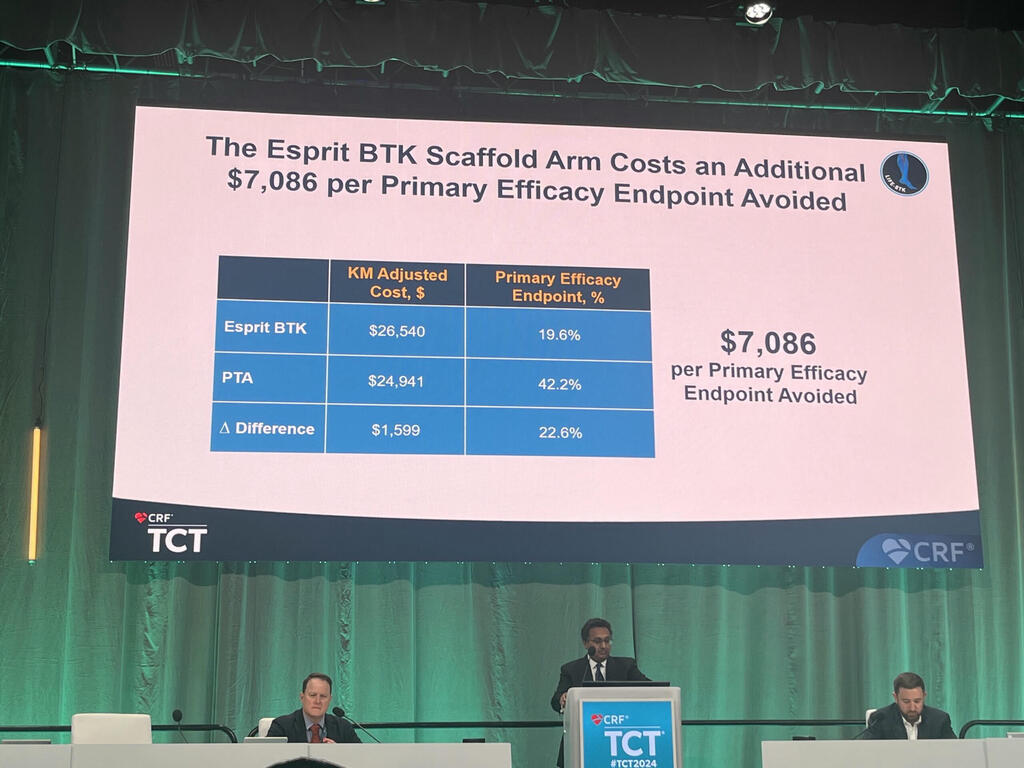
TCT 2024: Drug-eluting resorbable scaffold proves cost effective at one year in LIFE-BTK analysis
cardiovascularnews.com
Dec. 7, 2024, 6:27 a.m.
Using the most conservative delta between the two groups in terms of the primary efficacy endpoint at 365 days, not inclusive of the treatment window, the Esprit BTK scaffold costs an additional US$7,086 per primary efficacy endpoint avoided. This led Parikh to report that the Esprit BTK scaffold achieves a 64% probability of cost-effectiveness at a US$10,000 willingness-to-pay threshold compared to angioplasty. As a result of this, he averred: “The use of Esprit BTK at one year alone is likely to be cost effective.”
Share on

Camouflaging endovascular stents with an endothelial coat using CD31 domain 1 and 2 mimetic peptides
jvsvs.org
Sept. 2, 2024, 3:07 p.m.
Using peptides replicating the membrane-distal portion of CD31, which is prominently exposed on the inner side of healthy vessels, can simulate the surface of a healthy endothelium. This approach effectively prevents the deposition and activation of blood platelets and leukocytes, which are abnormally prolonged by the presence of a foreign body at sites of endovascular stent implantation, and promotes the acquisition of physiological phenotype by the ECs covering the surface.
Share on
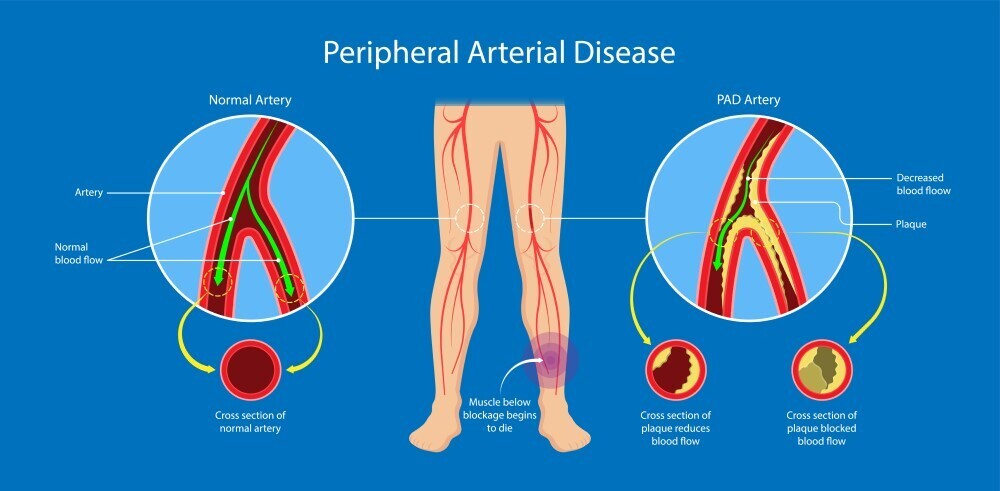
New Horizons in Peripheral Artery Disease
academic.oup.com
Aug. 15, 2024, 10:45 a.m.
Peripheral artery disease (PAD) is the lower limb manifestation of systemic atherosclerotic disease. PAD may initially present with symptoms of intermittent claudication, whilst chronic limb-threatening ischaemia (CLTI), the end stage of PAD, presents with rest pain and/or tissue loss. PAD is an age-related condition present in over 10% of those aged ≥65 in high-income countries. Guidelines regarding definition, diagnosis and staging of PAD and CLTI have been updated to reflect the changing patterns and presentations of disease given the increasing prevalence of diabetes.
Share on
New horizons in the treatment of lower extremity arterial disease
www.escardio.org
Aug. 15, 2024, 10:44 a.m.
Despite medical and technical progress, therapy of lower extremity arterial disease (LEAD) remains limited by subsequent disease progression and ultimate limb ischaemia. Even today, interventional and surgical revascularisation does not enable long-term success in all patients, and/or may not be technically feasible in end-stage LEAD anymore. Subsequent intractable claudication, impaired quality-of-life and work capacity, limb loss, and associated morbidity and mortality have thus generated impetus for alternative therapies.
Share on
Humacyte's ATEV granted US FDA Regenerative Medicine Advanced Therapy designation for advanced PAD
vascularnews.com
Aug. 15, 2024, 10:41 a.m.
Humacyte recently announced it has been granted Regenerative Medicine Advanced Therapy (RMAT) designation from the US Food and Drug Administration (FDA) for its investigational acellular tissue engineered vessel (ATEV), designed to treat patients with advanced peripheral arterial disease (PAD). This RMAT designation was granted at the same time as the FDA cleared a new Investigational New Drug (IND) application for the PAD indication for ATEV, formerly referred to as the human acellular vessel (HAV).
Share on
A subpopulation of tissue remodeling monocytes stimulates revascularization of the ischemic limb
www.science.org
Aug. 14, 2024, 8:07 a.m.
Despite decades of effort aimed at developing clinically effective cell therapies, including mixed population mononuclear cells, to revascularize the ischemic limb, there remains a paucity of patient-based studies that inform the function and fate of candidate cell types. In this study, we showed that circulating proangiogenic/arteriogenic monocytes (PAMs) expressing the FcγIIIA receptor CD16 were elevated in patients with chronic limb-threatening ischemia (CLTI), and these amounts decreased after revascularization. Unlike CD16-negative monocytes, PAMs showed large vessel remodeling properties in vitro when cultured with endothelial cells and smooth muscle cells and promoted salvage of the ischemic limb in vivo in a mouse model of hindlimb ischemia. PAMs showed a propensity to migrate toward and bind to ischemic muscle and to secrete angiogenic/arteriogenic factors, vascular endothelial growth factor A (VEGF-A) and heparin-binding epidermal growth factor.
Share on

Camouflaging Endovascular Stents with an Endothelial Coat Using CD31 Domain 1 and 2 mimetic Peptides
jvsvs.org
Aug. 5, 2024, 11:36 a.m.
CD31-coated surfaces promoted the acquisition of a physiologic endothelial cell phenotype and rapid coverage at a significantly higher extent as compared to bare metallic surfaces in vitro. Nitinol and cobalt-chromium stents coated with CD31-mimetic peptides were rapidly integrated within the arterial wall in vivo without thrombo-inflammatory signs up to 30 days after implantation.
Share on
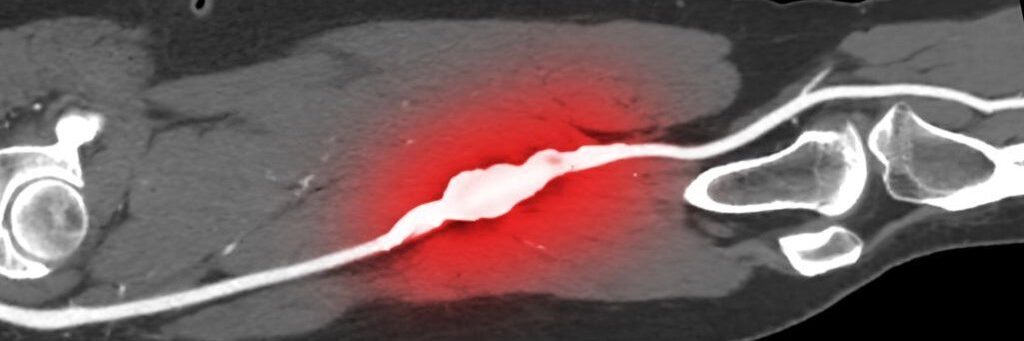
Comparative effectiveness of endovascular treatment modalities for de novo femoropopliteal lesions at long-term follow-up
www.sciencedirect.com
Aug. 5, 2024, 11:01 a.m.
DES was the best regarding 3-year primary patency, TLR, mortality, and 5-year TLR. DCB was the best regarding 3- and 5-year major amputation, and 5-year primary patency. DES and DCB should be given priority in treating de novo femoropopliteal lesions.
Share on
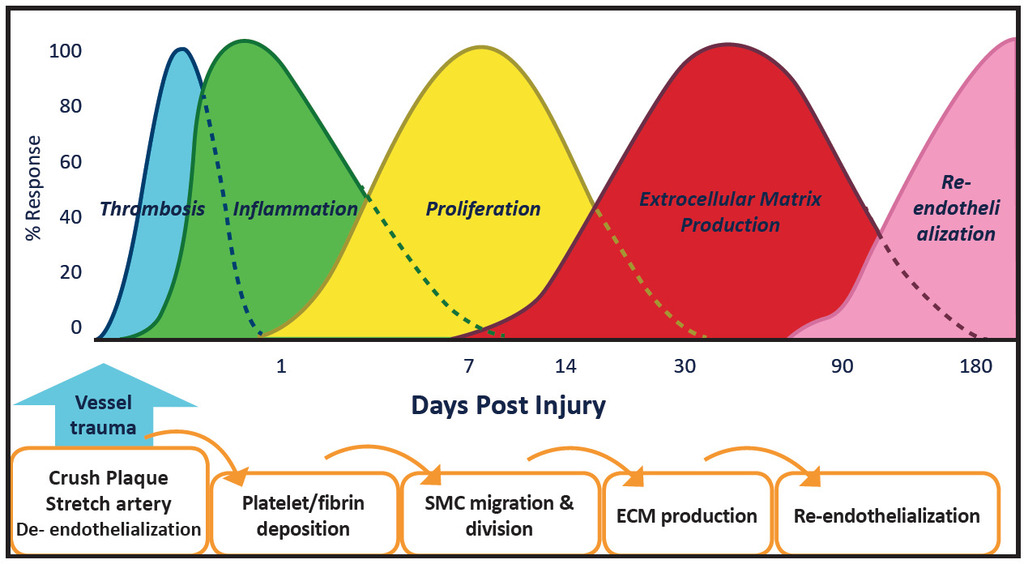
Combining a Drug-Coated Balloon and a Bare-Metal Stent: The REsponse Adapted Combination Therapy (REACT) Strategy
evtoday.com
July 11, 2024, 2:20 p.m.
Although evidence suggests that the performance of DCBs is independent of lesion complexity, there continues to be a bailout stent ratio > 40% in long lesions (> 20 cm), severely calcified lesions, and a high number of chronic total occlusions. In arteries that are obstructed by overwhelming atherosclerotic plaque deposition, balloon angioplasty increases the vessel lumen through uncontrolled dissection, resulting in longitudinal tears and creating tissue flaps with varying degrees of severity. Additional data also suggest that untreated dissections following plain old balloon angioplasty (POBA), including non–flow-limiting dissections, are associated with reduced patency.
Share on
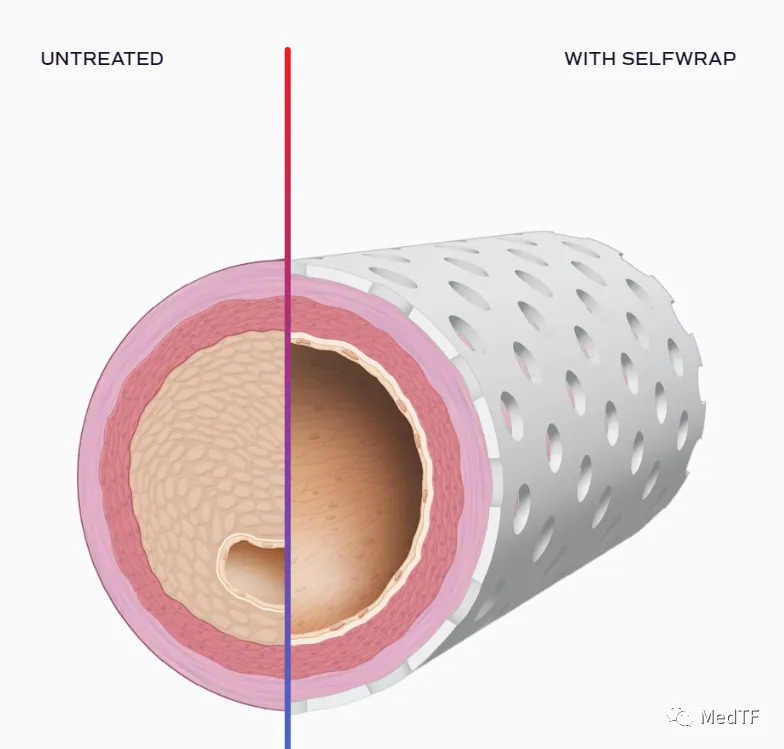
SelfWrap: Absorbable Extravascular Stent Closes Nearly $150 Million in Financing
mp.weixin.qq.com
July 8, 2024, 10:49 p.m.
VenoStent has developed an absorbable extravascular stent, SelfWrap, which not only retains the function of a non-absorbable extravascular stent like the VasQ, but can also be absorbed by the human body, and more importantly, it can be personalized for the patient to better meet his or her needs. Due to its technological innovation, SelfWrap received FDA Breakthrough Device designation in 2022 and was approved by the FDA for an IDE study last year.
Share on

Evolving Strategies for Use of Phytochemicals in Prevention and Long-Term Management of Cardiovascular Diseases (CVD)
www.mdpi.com
June 10, 2024, 6:37 a.m.
Cardiovascular diseases have been primarily characterized by the dysfunction of the myocardium as the primary and major cause of morbidity and mortality and have shown an increased rate across the globe. Both experimental and clinical studies show that the activation of inflammatory responses, myocardial necrotic, apoptotic, and autophagic processes substantially aggravate cardiac dysfunction, leading to the development of heart failure, ventricular arrhythmias, and sudden cardiac death.
Share on
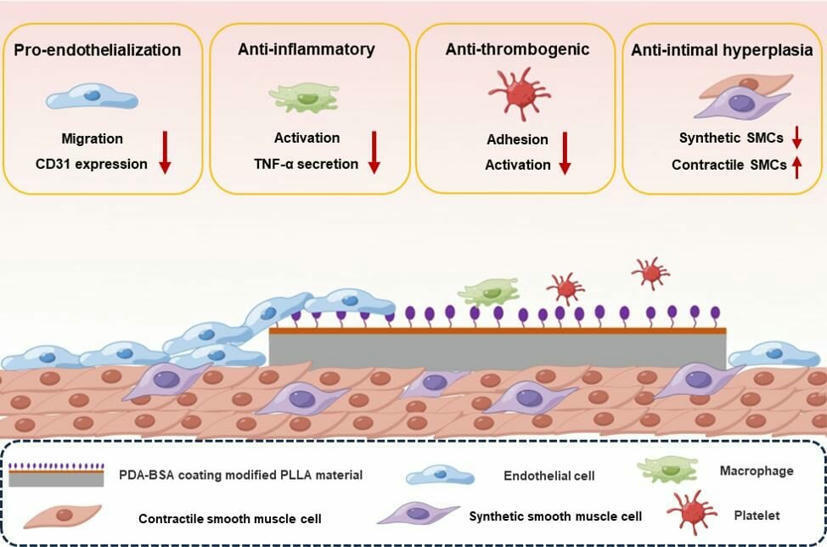
Biocompatibility of PLLA stents must be urgently improved
www.dovepress.com
June 10, 2024, 6:24 a.m.
Poly-L-lactic acid (PLLA) stents have broad application prospects in the treatment of cardiovascular diseases due to their excellent mechanical properties and biodegradability. However, foreign body reactions caused by stent implantation remain a bottleneck that limits the clinical application of PLLA stents. To solve this problem, the biocompatibility of PLLA stents must be urgently improved. Albumin, the most abundant inert protein in the blood, possesses the ability to modify the surface of biomaterials, mitigating foreign body reactions—a phenomenon described as the “stealth effect”.
Share on

New Guidelines on Peripheral Artery Disease Issued by American Heart Association, American College of Cardiology and Leading Medical Societies
www.dicardiology.com
May 27, 2024, 5:35 p.m.
A new joint guideline from the American Heart Association (AHA), the American College of Cardiology (ACC) and nine other medical societies reports early diagnosis and treatment of peripheral artery disease is essential to improve outcomes and reduce amputation risk, heart attack, stroke and death for people with Peripheral Artery Disease (PAD).
Share on
R3 Vascular raises $87M to test its bioresorbable, below-the-knee stent
www.fiercebiotech.com
May 20, 2024, 11:48 a.m.
The company said the proceeds would support its pivotal clinical trial in patients with severe vessel blockages below the knee, including people facing the potential loss of the limb, as well as help scale up its manufacturing base.
Share on

Drug-Eluting Resorbable Scaffold versus Angioplasty for Infrapopliteal Artery Disease
www.nejm.org
May 14, 2024, noon
Among patients with CLTI due to infrapopliteal artery disease, the use of an everolimus-eluting resorbable scaffold was superior to angioplasty with respect to the primary efficacy end point.
Chocolate Touch drug-coated angioplasty balloon for treatment of PAD receives FDA approval
vascularnews.com
May 14, 2024, 11:58 a.m.
The Chocolate Touch showed statistically superiority in its primary efficacy endpoint of 12-month true DCB success—a measure of the target vessel remaining patent without the need for bail-out stenting. Primary patency by Kaplan-Meier (KM) estimate was 83.3% for the Chocolate Touch and 73.0% for Lutonix DCB at 12 months.
Share on

Micro Medical Solutions completes enrolment for STAND MicroStent trial
vascularnews.com
May 14, 2024, 11:55 a.m.
STAND (A Clinical Evaluation of the MicroSTent PeripherAl Vascular SteNt in subjects with Arterial Disease Below the Knee) enrolled its final patient earlier this month marking the completion of the study. MicroStent is a vascular stent specifically designed to achieve and maintain tibioperoneal arterial patency improving blood flow and wound healing for below-the-knee amputation reduction in patients with CLTI resulting from progressive peripheral artery disease (PAD).
Share on