
Abbott TMVR System Receives FDA Approval
www.dicardiology.com
June 3, 2025, 8:25 a.m.
The Tendyne system replaces mitral valves that are not functioning properly due to a buildup of calcium in the base of the valves, known as severe mitral annular calcification.
Share on

Balloon-assisted procedure found safe and effective for patients undergoing transcatheter mitral valve replacement
medicalxpress.com
May 5, 2025, 6:18 a.m.
New data from a large, international registry showed balloon-assisted anterior mitral leaflet modification (BATMAN) was safe, effective, and resulted in shorter procedure times among patients undergoing transcatheter mitral valve replacement (TMVR). The data were presented today as late-breaking clinical research at the Society for Cardiovascular Angiography & Interventions (SCAI) 2025 Scientific Sessions.
Share on
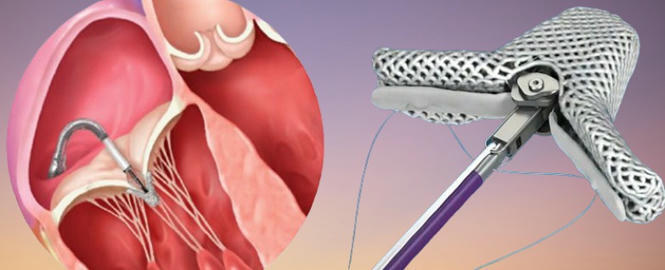
Right Ventricular Function Predicts Outcomes After PMVR
www.physiciansweekly.com
April 21, 2025, 8:15 a.m.
Mitral regurgitation (MR) is a prevalent valvular heart condition often associated with poor clinical outcomes, especially when left untreated or inadequately managed. While percutaneous mitral valve repair (PMVR) with the MitraClip system, in conjunction with guideline-directed medical therapy, has demonstrated clinical and prognostic benefits in select patients with symptomatic severe MR, a considerable proportion of individuals continue to experience major adverse cardiovascular events (MACE)—including death or heart failure-related hospitalizations, within the first year following the procedure. Consequently, early identification of patients who are unlikely to respond favorably remains a critical clinical goal.
Share on

Common Imaging Test Uses AI to Help Identify Heart Valve Disease
www.dicardiology.com
April 19, 2025, 5 p.m.
An artificial intelligence (AI) program trained to review images from a common medical test can detect early signs of tricuspid heart valve disease and may help doctors diagnose and treat patients sooner, according to research from the Smidt Heart Institute at Cedars-Sinai.
Share on

Early feasibility study of Siegel 8Fr TAVI valve launches
cardiovascularnews.com
April 19, 2025, 4:55 p.m.
The Siegel valve encompasses an 8Fr delivery sheath allowing less invasive procedures and broader patient access, a Nickel-free valve allowing treatment, lack of foreshortening and intrinsic commissural alignment and dry porcine pericardial leaflets with anti-calcification treatment with the valve pre-mounted on the balloon.

DurAVR transcatheter heart valve reaches 100 patients
cardiovascularnews.com
April 19, 2025, 4:52 p.m.
The 100 cases include de novo aortic stenosis, valve-in-valve (ViV) patients and complex anatomies such as bicuspid aortic valve patients. Sixty five patients have successfully completed the primary endpoint measures of safety and efficacy, including haemodynamic benefit at 30-days post implant.

Edward Lifesciences’ Sapien M3 transfemoral mitral valve system gains CE mark
cardiovascularnews.com
April 19, 2025, 4:46 p.m.
Edwards Lifesciences has announced that the company’s Sapien M3 mitral valve replacement system has received CE mark for the transcatheter treatment of patients with symptomatic (moderate-to-severe or severe) mitral regurgitation (MR) who are deemed unsuitable for surgery or transcatheter edge-to-edge (TEER) therapy.

Master & Fellow - Pipeline™ Vantage with Shield Technology™ for intracranial aneurysms by MEDTRONIC
masterandfellow.com
March 26, 2025, 4:53 p.m.
Pipeline™ is the reference in flow diversion, with more than 10 years of experience and a very high number of clinical studies. These devices changed the treatment of aneurysms. By diverting flow away from the aneurysm neck and reconstructing the parent artery, they allow the flow to reapir its natural course.
Share on

Cardiac Dimensions secures $53M bringing a new dimension to heart failure treatment
techfundingnews.com
March 24, 2025, 8:46 a.m.
Cardiac Dimensions, a leader in minimally invasive heart failure treatments, has raised $53M in an oversubscribed Series E funding round to advance its minimally invasive Carillon Mitral Contour System for heart failure treatment and expand global commercialisation efforts. Led by Ally Bridge Group, the investment also saw strong participation from existing backers, reinforcing confidence in the company’s innovative Carillon Mitral Contour System.
Share on
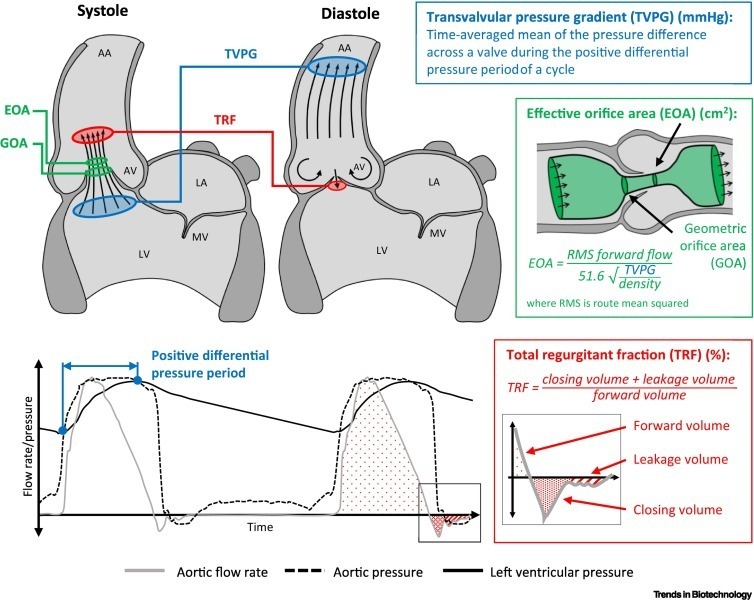
3D printing of heart valves
www.cell.com
March 22, 2025, 11:15 a.m.
The manufacture of artificial heart valves is very challenging owing to their stringent functional requirements. The latest advances in 3D printing technologies hold great promise to revolutionise the manufacture of artificial heart valves. Recent 3D printing innovations go far beyond the fabrication of anatomical moulds, and unlock unprecedented opportunities to manufacture exquisite architectures, hierarchical bioprinting of cells, complex multi-material assembly, and the creation of biomimetic structures. This provides new avenues to enhance the functionality of artificial heart valves with respect to hydrodynamics, durability, biocompatibility, and the ability to be implanted via a minimally invasive transcatheter procedure.
Share on
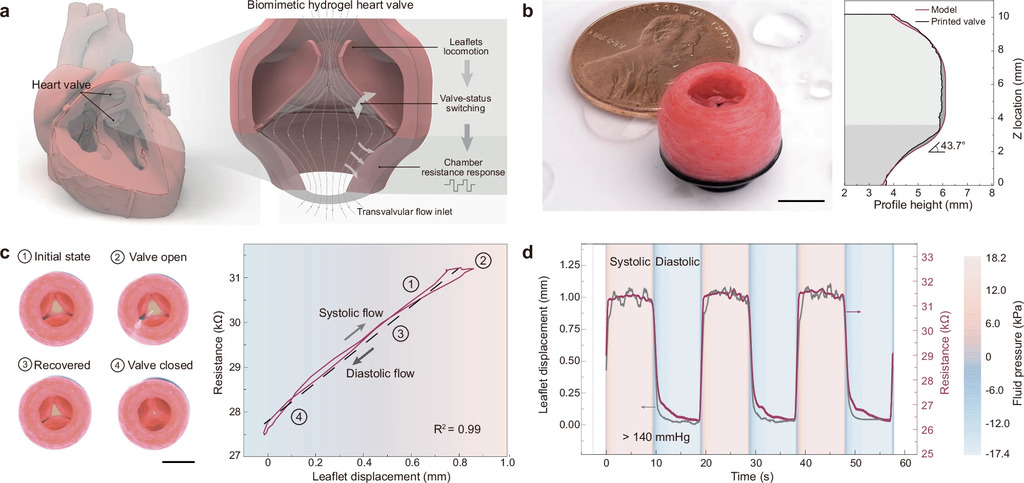
Multimaterial cryogenic printing of three-dimensional soft hydrogel machines
www.nature.com
March 22, 2025, 11:13 a.m.
Hydrogel-based soft machines are promising in diverse applications, such as biomedical electronics and soft robotics. However, current fabrication techniques generally struggle to construct multimaterial three-dimensional hydrogel architectures for soft machines and robots, owing to the inherent hydrogel softness from the low-density polymer network nature. Herein, we present a multimaterial cryogenic printing (MCP) technique that can fabricate sophisticated soft hydrogel machines with accurate yet complex architectures and robust multimaterial interfaces.
Share on

Formulation and Evaluation of PVA/Gelatin/Carrageenan Inks for 3D Printing and Development of Tissue-Engineered Heart Valves
advanced.onlinelibrary.wiley.com
March 22, 2025, 11:09 a.m.
Congenital and acquired valvular heart diseases (VHDs) are significant causes of mortality worldwide. With valve replacement being the primary solution for VHD, current options display shortcomings, including calcification, thrombogenicity, and hemodynamic alteration, leading to repetitive surgeries. Tissue engineering, however, has shown great potential for fabricating heart valves (HVs) with fewer complications. Here, a series of inks are developed, combining poly(vinyl alcohol), gelatin, and carrageenan for 3D printing of tissue-engineered heart valves (TEHVs). The inks/hydrogels are investigated to characterize their physico-chemical, morphological, mechanical, and rheological characteristics. In vitro and in vivo biocompatibility, immune response, hemolysis, and thrombogenicity of the inks/hydrogels are also evaluated.
Share on

Remplacement percutané de la valve mitrale : le concept prometteur de la bioprothèse en cage
francais.medscape.com
March 11, 2025, 10:50 a.m.
Le dispositif a l’avantage de pouvoir bénéficier aux patients présentant des calcifications sur les feuillets de la valve mitrale. « Des patients inéligibles au TEER [réparation transcathéter bord à bord de type Mitraclip, ndr] et pour lesquels il n’y a pas vraiment d’option », a rappelé le Dr Sebastian Ludwig (University Heart & Vascular Center Hamburg, Hambourg, Allemagne), lors d’un échange en fin de présentation.
Share on
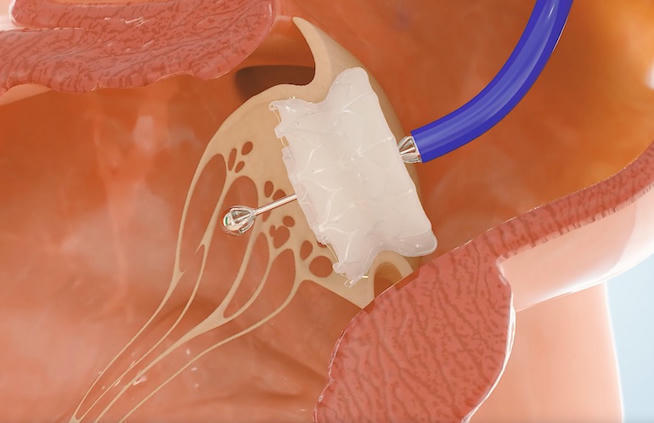
Capstan Medical completes robotic catheter mitral valve replacement
www.therobotreport.com
March 10, 2025, 8:21 a.m.
Capstan Medical today said it has successfully completed its first in-human, robot-assisted transcatheter mitral valve replacements. The Santa Cruz, Calif.-based company claimed that it was “the first time a minimally invasive robotic structural heart platform has been used in clinical practice, successfully delivering a novel mitral valve into two human patients.”
Share on
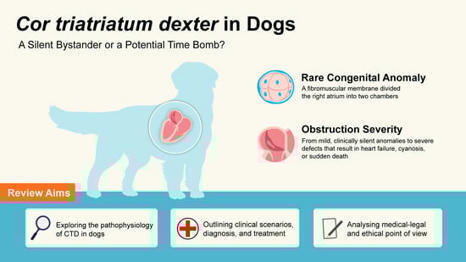
Cor triatriatum dexter in Dogs: A Silent Bystander or a Potential Time Bomb?
www.mdpi.com
Feb. 17, 2025, 11:10 a.m.
Cor triatriatum dexter (CTD) is an uncommon congenital anomaly consequent to an abnormal separation of the atrium during embryological development, determining the persistence of the fibromuscular membrane dividing the right atrium. Based on the severity of the obstruction determined by the membrane, the clinical signs may be silent or appear as heart failure, with consequent ascites, and if there are other associated congenital cardiac anomalies, they could manifest as cyanosis or sudden death. The aim of the present review is to give a professional perspective, considering the defect from different points of view.
Share on

Making Heart Valves in Minutes
mp.weixin.qq.com
Feb. 13, 2025, 9:16 a.m.
Researchers at Harvard University have developed a technique that can make a biomaterial heart valve in minutes. The method is called “Focused Rotary Jet Spinning,” and the researchers describe it as “a marshmallow machine with a hairdryer at the back.” Basically, this technique uses air spray to direct the polymer fibers to a heart valve-shaped frame. This forms a porous scaffold that allows heart cells to enter and grow. These formed structures also have mechanical properties that serve as unidirectional valves within the heart. These scaffolds contain nanoscale signals that stimulate cell entry and proliferation, with the ultimate goal of gradually replacing biological material scaffolds by cells to achieve heart valve regeneration.
Share on

TCT 2024: Adjustable valve replacement system wins Shark Tank innovation prize
cardiovascularnews.com
Dec. 7, 2024, 6:22 a.m.
The Valysnc system comprises two highly compliant balloons, made of a flexible deformable material that can expand to fit the surrounding valve anatomy. The system is designed to be delivered transeptally, with an atrial balloon expanded and lowered onto supra-annular plane of the mitral valve. Afterwards a second balloon is secured in the ventricle, held in place by a series of barbs or arms.
Share on
TCT 2024: Non-invasive ultrasound therapy device shows promise in calcific aortic stenosis
cardiovascularnews.com
Dec. 7, 2024, 6:19 a.m.
Valvosoft is designed to non-invasively restore leaflet mobility in a stenotic aortic valve and widen the valve opening to relieve patient heart failure symptoms. High-intensity focused ultrasound waves micro-fracture calcification embedded in aortic valve leaflets without damaging tissue. The treatment is designed to be repeatable over time, as needed, to manage disease progression.
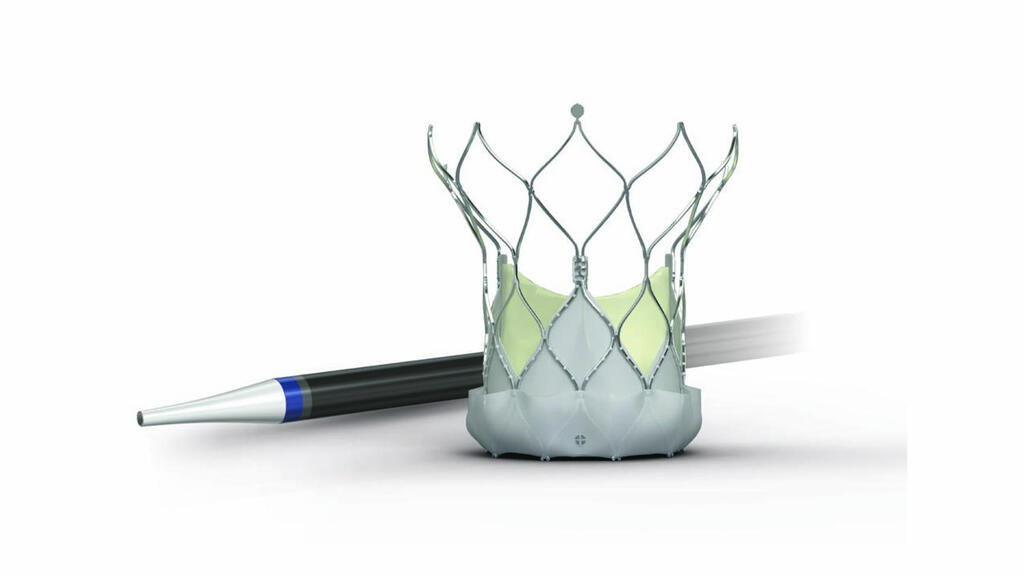
Abbott launches its largest Navitor Vision valve size in UK
cardiovascularnews.com
Dec. 7, 2024, 6:05 a.m.
The company has described this the largest valve size, supplied through the smallest delivery system available on the NHS, offering flexibility, accuracy, and stability in a large TAVI valve platform and is being used at King’s College Hospital in London.
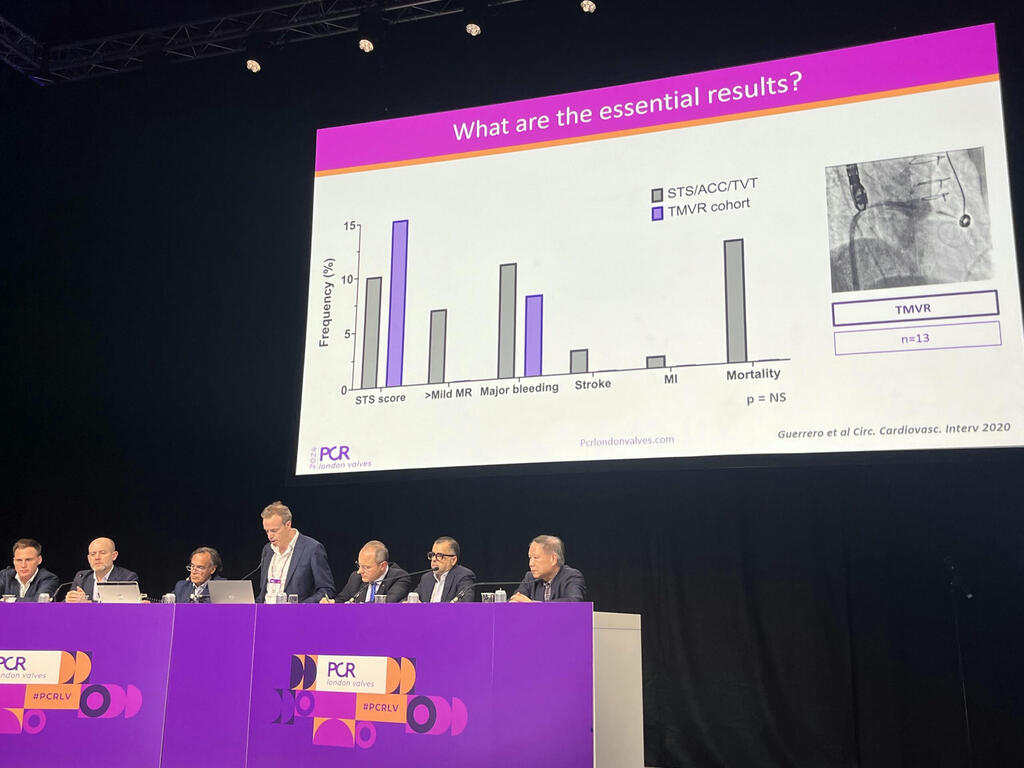
PCR London Valves 2024: Live cases at cardiology conferences “do not compromise patient safety”
cardiovascularnews.com
Dec. 7, 2024, 6:03 a.m.
Whether the educational opportunity that is afforded by a live case justifies any potential safety risk to the patient continues to be a source of debate, Allen said, prompting the analysis looking at procedural safety among 115 live cases. Their researched comprised transcatheter aortic valve implantation (TAVI) and mitral and tricuspid procedures from three high-volume centres over a 14-year period. Researchers compared safety outcomes from the live cases to corresponding clinical trials and real-world daily practice.
Share on